Scientists create anti-ageing gene therapy based on CRISPR
Kat7 gene inactivation promotes longevity and reinvigorates prematurely ageing cells.
Key takeaways
- Wang et al. conducted a genome-wide screen based on CRISPR-Cas9. The test was developed to identify genes that could impact cellular senescence.
- They identified KAT7 promotes senescence in human adult stem cells.
- Inactivation of KAT7 reinvigorated prematurely ageing progeroid human cells. Furthermore, it extended the lifespan of mice.
It is unknown how cellular senescence, a perpetual state of growth arrest, occurs in the body. Cellular senescence is known to advance ageing, so our comprehension of what exacerbates cellular senescence is vital in developing interventions to promote longevity and anti-ageing.
Wang and colleagues from the Chinese Academy of Sciences in Beijing recently published results in the journal Science Translational Medicine. The results were based on a test where they mined for genes that could impact cellular senescence. They took DNA from human cells modelling ageing with the gene-editing tool CRISPR for genes whose deficiency reduced cellular senescence. The screening resulted in KAT7, leading to the rejuvenation of prematurely ageing cells when inactivated. When the scientists disabled KAT7 in mice following normal ageing patterns and prematurely, the mices’ lifespan was extended.
These are promising results regarding KAT7 and its capabilities for developing anti-ageing interventions. Furthermore, it adds to the existing evidence that CRISPR-based gene editing can promote further longevity. “Our study adds another example showing the possibility of using gene therapy for antagonising ageing and ageing-related disorders,” said Wang and colleagues.
Alleviating human cellular senescence
Ageing is an apparently unstoppable process that leads to functional decline in almost every organism. Cellular senescence has emerged as both a fundamental element in anti-ageing and a main player in the ageing process. Senescent cells amass in tissues as time goes on, developing natural features of organismal ageing and adding to age-related conditions like Alzheimer’s and arthritis.
Senescent cells aren’t only indicative of ageing: they play an important role in organismal ageing. For example, senescent cells gradually amass in the deteriorated liver, whereas alleviating the liver of the senescent cells reduces the development of hepatic steatosis – fatty liver disease. So, reversing or delaying cellular senescence may create a new therapeutic approach for fighting age-related conditions.
While we’re learning more about what happens to the body as we get older, most of what we understand about the genes that play an important role in ageing originates from experiments using short-lived model organisms, including flies, fish, worms and yeast. Therefore, it is important to continue researching genetic programs that determine human ageing.
Tests identify new human senescence-promoting genes
Wang and colleagues utilised the genetic engineering tool CRISPR to eliminate small amounts of DNA to identify genes affecting ageing using two types of human cells displaying increased senescence. These cells contained disease-producing mutations that cause premature ageing diseases Hutchinson-Gilford progeria syndrome (HGPS) and Werner syndrome (WS). The scientists identified genes whose deficiency alleviated cellular senescence, including KAT7, which was prominent in both HGPS and WS ageing human cell models.

(Wang et al. 2021 | Sci Transl Med) KAT7 inactivation by CRISPR gene editing technique reinvigorates Werner syndrome adult stem cells. KAT7 deficiency (sg-KAT#1 and sg-KAT#2) dramatically enhanced the proliferative potential of Werner Syndrome (WS) modelling adult stem cells without KAT7 deficiency (sg-NTC) to levels of non-mutated, typical cells (WT) (Left). KAT7 deficiency alleviated various premature ageing issues previously identified in WS adult stem cells. This is evidenced in the following findings: (i) enhanced percent of replicating (Ki67-positive) cells (middle); (ii) reduced percentage of senescent (SA-β-gal-positive) cells (right).
To determine whether KAT7 deficiency reduced or even alleviated senescence, Wang and colleagues zeroed-in on KAT7 for gene silencing by CRISPR in HGPS, WS and replicative-senescent adult stem cells. They concluded that KAT7 ablation in each model alleviated ageing features and improved proliferative potential. Moreover, the ablation of KAT7 alleviated senescence models of oxidative stress, UV and cancer. These results indicate that KAT7 depletion reduces senescence in a variety of biological contexts.
Finally, Wang and colleagues tested the anti-ageing CRISPR gene therapy method in living organisms. So, the team tested the impact of inactivating KAT7 with CRISPR in mice aged prematurely and naturally. Utilising viruses to deliver CRISPR components to inactivate KAT7, administered intravenously, the team found that it alleviated liver ageing and liver cell senescence as well as extended the lifespan of mice aged both prematurely and naturally.
While promising, these impacts were not realised in other organs because the team only found the gene-editing of KAT7 occurred in the liver most likely because of the viral treatment method. There will have to be further studies conducted to examine the consequences of targeting KAT7 in specific organs or more cell types to determine the safety and function scope of KAT7 intervention.
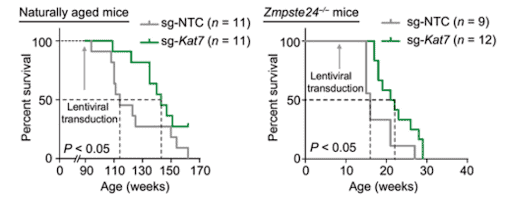
(Wang et al. 2021 | Sci Trans Med) Gene therapy targeting KAT7 extends lifespan and health span in naturally aged mice. Survival curve (left) of mice aged naturally infected virus-holding CRISPR targeting components for KAT7 (sg-Kat7; green) or non-specific DNA (sg-NTC; grey) at 20 months. Survival curve (right) of prematurely ageing (Zmpste24-/-) mice infected with CRISPR components targeting non-specific DNA (sg-NTC) virus or KAT7 (sg-Kat7).
A potential anti-ageing treatment?
“Our study adds a layer of complexity to KAT7 function by revealing its important role in ageing biology and pinpointing KAT7 as a new target for delaying ageing and treating ageing-associated disorders,” said the team. “In addition to unravelling the role of KAT7 in mediating ageing, our screen identified additional senescence genes that might be targeted to ameliorate ageing-related processes.”